CRC Handbook of Free Radicals and Antioxidants ,vol 1 (1989), p209-221.
Peter H. Proctor, PhD, MD
Oxygen? "I rarely use it myself, sir. It promotes rust." Robby the Robot, Forbidden Planet(1956)
( Earlier versions: Radical Disease, 1972and Radical Disease, 1984)
Free radical ( "Redox:" ) signaling: Conference on Active Oxygen and Medicine, Honolulu, March,(1979). Abstract .
Indirect evidence suggests that free radicals and excited-state species play a key role in both normal biological function and in the pathogenesis of certain human diseases. For example, generation of activated species by inflammatory cells is a major microbiocidal mechanism and may also mediate important components of the inflammatory response. Activated processes may also be key components in the toxicity of many drugs, in aging, and in carcinogenesis. They may also figure in the etiology of certain ocular, neurological, and psychiatric diseases.
The evidence for a role for
electronically activated species in human disease
has long been prevalent. For example, Darwin repeats
the well-known observation that white, blue-eyed
cats are usually deaf. Similarly, the relationship
between pigmentary abnormalities and human deafness
(for example, in Waardenberg's or Usher's
syndromes) is commonplace in audiology(4). Likewise,
physicians have long recognized the association
between radical-generating metals such as copper or
iron and fibrotic changes, e.g., interocular
fibrosis in vitreous chalcosis and liver cirrhosis
in Wilson's disease and Hemochromatosis.
Further, free radicals and other activated species are so difficult to measure under biological conditions that the evidence for their role in any biological process - much less a human disease state - is normally indirect and circumstantial. This flawed scientific basis often results in heated controversy over methodology, results, and conclusions. Even less should be expected of the clinical evidence. Nonetheless, there is significant circumstantial evidence that active oxygen (Figures 1 and 2) is involved in some of the most fundamental mechanisms in pathogenesis and in the etiology of many human diseases.
Figure 1 The Active Oxygen System
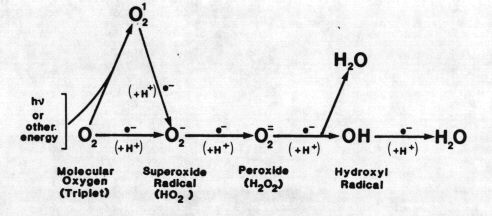
FIGURE 1. The active oxygen system. Molecular oxygen is reduced to water in four single-electron steps. Reduction of nonradical forms of oxygen is a " forbidden " process and thus usually involves spin-orbit coupling by a heavy metal or a halide or excitation to singlet state. An example is Fenton's reaction, the reduction of peroxide to water and hydroxyl radical by ferrous iron. Hydroxyl radical is one of the most powerful oxidizing agents known. Simply put, reducing agents act as prooxidants by reducing nonradical forms of oxygen to radical forms, usually with heavy atom involvement. Similarly, they can act as antioxidants by reducing radical forms of oxygen, by terminating radical chain reactions, or by, for example, reducing hydroperoxides. This dual property can be of great significance. For example, in humans uric acid is probably the primary extracellular antioxidant. On the other hand, a Fenton-type reaction of phagocytized urate with granulocyte-produced peroxide may contribute to the etiology of gout.
Figure 2: Neuromelanins
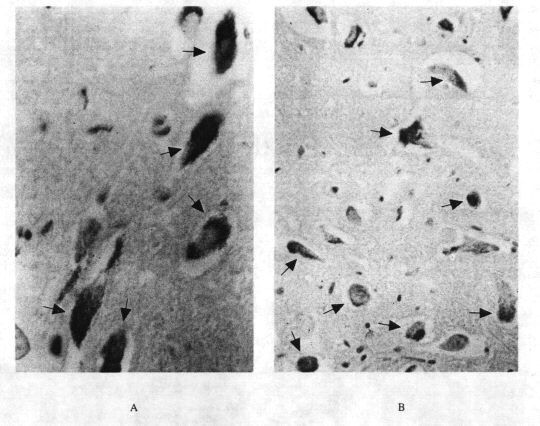
FIGURE 2. Neuromelanin. A:
Dopaminergic pigmented neurons in
pars compactaof
substantia nigraand B:
Noradrenergic pigmented neurons
from
locus ceruleus. ( Autopsies
by the author).
Most, if not all, central
catecholaminergic neurons contain a
stable free radical, melanin.
Specific dying-off of pigmented
neurons in the
substantia nigrais the
apparent cause of Parkinson's
disease. Dopaminergic neurons may
also be concerned in schizophrenia
and in various movement disorders (
e.g., choreoathetosis in the
Lesch-Nyhan syndrome ).
Noradrenergic neurons may figure in
endogenous depression and
Altzheimer dementia. The function,
if any, of melanin in such neurons
is unknown but it may be related to
its antioxidant and semiconductor
properties. G. C. Cotzias on
neuromelanins: " The
neuromelanin granule may be the
secret key to the understanding of
Parkinsonism. I don't believe
God put the melanin granule in the
central nervous system for nothing.
It must be doing something.
Something big... "
Later note:
Go here
and
here
for examples of the
drop-out of
melanin-containing neurons in
Parkinson's disease.
Also,
melanin-bound
iron,
increases in
Parkinsonism-- vis, the
parkinsonian-like
symptomology occasionally
found in
hemochromatosis.
Go here
for an example of the
antioxidant properties of
melanin.
The evidence for a role in
disease is of several types.
For example, many human
diseases present with
increased production of
activated species or with
increased levels of
radical-generating
substances. Examples include
granulocyte activation in
inflammation or copper in
Wilson's disease.
Additionally, the progression
of many diseases may be
modulated pharmacologically
by ectopically administered
superoxide dismutase (SOD),
catalase, or free radical
scavengers. Finally, many
such diseases are also
associated with one or more
characteristic symptoms
(Table 1).
The Oxygen-Dependent
Microbiocidal System
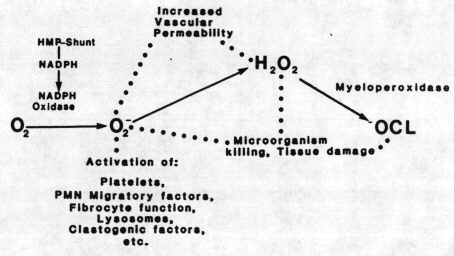
Figure 3: The
Role of Active
Oxygen Species in
Inflammation
FIGURE 3.
Role of
active oxygen
species in
inflammation.
Inflammatory
cells (
granulocytes,
macrophages,
some
T-lymphocytes,
etc. )
produce
active
species of
oxygen as
part of the
microbiocida1/citocidal
system. In
turn, active
oxygen
species can
modulate
specific
elements of
the
inflammatory
response in
vitro.
Examples
include
protein
immunomodulator
substances
such as
granulocyte
migratory
factors,
prostaglandins,
cyclic
nucleotides,
as well as
formed
elements such
as platelets.
Which, if
any, of these
are relevant
to the
in
vivosituation
is unknown.
Antioxidant
Defenses
and Solid
State
Defenses
Biological
systems
protect
themselves
against
the
damaging
effects
of
activated
species
by
several
means
(21-22).
These
include
free
radical
scavengers
and chain
reaction
terminators:
enzymes
such as
SOD,
catalase,
and the
glutathione
peroxidase
system;
and
"
solid-state
"
defenses
such as
the
melanins.
Chemical
antioxidants
act by
donating
an
electron
to a free
radical
and
converting
it to a
nonradical
form.
Likewise,
such
reducing
compounds
can
terminate
radical
chain
reactions
and
reduce
hydroperoxides
and
epoxides
to less
reactive
derivatives.
However,
chemical
antioxidant
defense
is a
double-edged
sword.
When an
antioxidant
scavenges
a free
radical,
its own
free
radical
is
formed.
Many
antioxidants
can act
as
pro-oxidants
by, for
example,
reducing
nonradical
forms of
oxygen to
their
radical
derivatives,
particularly
if redox
cycling
occurs.
The exact
mix of
pro- and
antioxidant
properties
of a
reducing
compound
is a
complex
interaction
involving
pH,
relative
reactivities
of
radical
derivatives,
availability
of metal
catalysts,
and so
forth.
For
example,
the anti-
or
pro-oxidant
properties
of
sulfhydryl
compounds
depend
upon pH
(29-31),
those of
beta
carotene
upon
oxygen
concentration
(69).
Likewise,
uric
acid,
probably
a
significant
antioxidantin
higher
primates
(32-36)
participates
in a
Fenton-type
reaction
with
peroxide(35,
a
property
which may
be
important
in the
etiology
of gouty
inflammatory
disease.
Similarly,
stable
radical
formers,
such as
the
melanins
or the
nitroxide
spin
labels,
scavenge
odd
electrons
to form
stable
radical
species,
thus
terminating
radical
chain
reactions.
Interestingly,
minoxidil,
noteworthy
because
of its
ability
to
stimulate
hair
growth
and
reverse
pattern
balding,
is a
nitroxide
closely
analogous
to
commonly
used spin
labeling
compounds.
Later
Note
:
Go
here
for a
discussion
of
how
free
radicals
may
modulate
hair
growth.
This
is
another
manifestation
of
free
radical
(
"
redox
"
)
signaling.
Also,
nitroxide
spin
labels
such
as
TEMPOL
are
potent
SOD-mimetics.
Both
spin
traps
and
spin
labels
have
significant
potential
drug
applications
for
most
of
the
diseases
considered
in
this
review.
Go
here
for
more
on
this.
Enzymatic
defenses
against
active
species
include
SODases,
catalases,
and
the
glutathione
reductase/peroxidase
system.
While
there
have
been
some
thoughtful
questions
raised,
SOD
appears
to be
one
of
the
most
specific
enzymes
known.
Inhibition
of a
biological
process
by
SOD
is
often
taken
as
putative
evidence
for a
role
for
superoxide
in
that
process.
However,
some
of
the
actions
of
SOD
appear
to be
due
to
peroxide
production
rather
than
superoxide
destruction
(4).
Most
recent
work
in
free
radical
defense
centers
on
chemical
antioxidants
and
enzymatic
defenses.
However,
a
third
class
of
mechanism,
solid-state
defense,
may
be at
least
as
important,
particularly
with
respect
to
human
disease.
In
solid-state
defense,
a
macromolecule
binds
a
radical-generating
compound,
deexcites
an
excited-state
species,
or
quenches
a
free
radical.
In
many
ways
the
internal
action
of
SOD
matches
this
definition.
However,
the
most
important
solid-state
defense
is
probably
the
black
pigment
melanin.
Melanin
is
also
important
because
it is
the
only
biological
polymer
which
is a
stable
free
radical
and
was
the
first
free
radical
established
in
biological
systems. In
the
same
manner,
a
visible
pigmentary
response
often
occurs
in
the
presence
of a
radical-dependent
process,
be it
a
dermal
inflammatory
process,
UV
light,
or
the
chronic
presence
of a
pro-oxidant
such
as
iron
in
hemochromatosis.
This
dermal
pigmentary
response
is
the
only
part
of
the
defensive
reaction
to
active
species
which
is a
clinically
apparent
symptom.
Thus,
it
forms
a
part
of
the
radical-dependent
symptom
complex
(Table
1)
and
represents
a
visible
outward
sign
of an
otherwise
invisible
active
process.
While
chemically
inert,
melanin
is a
very
active
"
amorphous
semiconductor
".
In
amorphous
semiconductors,
photon
or
electronic
energy
in
the
form
of
motions
of
electrons
is
very
strongly
coupled
to
molecular
vibrations
or
"
phonons
".
Heat
is
one
manifestation
of
phonon
energy,
while
sound
vibrations
are
another.
That
is,
in
the
melanins
electronic
or
excited-state
energy
readily
exchanges
with
vibrational
energy
in
the
form
of
heat
or
sound.
Such
seemingly
esoteric
considerations
may
explain
much
of
the
physical
properties
and
biological
functions
of
the
melanins
(35-41).
For
example,
the
melanins
are
black,
photoprotective,
and
nonfluorescent
because
most
photon
energy
(
e.g.,
from
light
or
chemically
produced
excited-state
species
)
absorbed
by
them
readily
converts
into
heat
(
36~37
).
This
likely
explains
the
presence
of
melanins
in
such
energy-transducing
areas
as
the
skin,
retina,
and
inner
ear.
Conversely,
rate
limitations
for
such
conversions
mean
that
the
melanins
may
themselves
be
toxic
to
the
cells
which
contain
them
by
electronically-activated
mechanisms
(4-9).
This
may
be
important
in
the
etiology
of
such
disorders
as
Parkinsonism,
senile
macular
degeneration,
and
senile
deafness
(4,9-27,41
).
Likewise,
the
ability
of
melanins
to
readily
convert
vibrational
energy
in
the
form
of
sound
into
electronic
energy
means
that
they
are
by
far
the
best
sound-absorbing
materials
known
(38).
This
may
account
for
their
presence
( as
protective
devices
? )
in
the
inner
ear
(4,9-27,41).
It is
also
relevant
to
the
well-known
association
between
pigmentary
abnormalities
or
the
presence
of
melanin-binding
drugs
in
deafness
(
e.g.,
Waardenburg's
syndrome
and
aminoglucoside
ototoxicity
), as
well
as
the
association
of
deafness
with
pigmentary
retinopathies
in
Usher's
syndrome
and
chloroquine
toxicity.
The
melanins
also
have
some
rather
exotic
electronic
properties.
For
example,
they
can
act
as
threshold
switches
and
can
store
electrical
energy
like
batteries(
39 ).
Such
properties
may
explain
the
presence
of
melanins
in
electrically
active
tissues
such
as
the
substantia
nigra,
where
it
may
play
a
role,
for
example,
in
Parkinson's
disease
(
Figure
2 ).
Later
note
:
By
nearly
a
decade,
melanin
was
the
first
organic
semiconductor
used
in
an
"
active
"
electronic
device
(
a
bistable
switch),
where
an
electric
field
controls
current
flow.
Bistable
switches
are
the
basic
unit
of
your
computer.
Since
the
"on"
state
of
this
switch
has
almost
metallic
electrical
conductivity,
melanin
is
also
the
first
organic
compound
shown
to
have
a
high-conductivity
state.
Melanins
are
polyacetylenes
and
vice
versa.
Much
to
our
surprise,
the
later
discovery
of
high
conductivity
produced
by
chemical
means
in
another
polyacetylene
(
i.e.,
another
"melanin"
)
won
the
2000
Nobel
Prize
in
Chemistry.
This
is
like
recognizing
simple
semiconductivity
in
silicon,
while
ignoring
its
previous
use
in
a
transistor.
Interestingly,
nearly
all
organic
semiconductor
devices
since
have
technically
used
"melanin"
as
their
active
element.
There
is
no
evidence
the
Nobel
Committee
was
aware
of
our
previous
work,
though
it
was
published
in
a
major
journal,
Science.
See
organicsemiconductors.com
for
the
details.
But
enough
complaints--
back
to
the
paper....
Melanin
also
forms
stable
free
radicals,
quenches
excited
states,
and
binds
radical-forming
agents
such
as
transition-series
metals.
All
likely
contribute
to
its
putative
role
in
antioxidant
defense.
On
the
other
hand,
the
ability
of
melanin
to
bind
toxic
radical-generating
agents
may
sometimes
be
detrimental,
as
in
chloroquine
retinopathy
and
aminoglycoside
ototoxicity
(41).
Finally,
melanin
can
function
as
an
efficient
S0Dase
and
may
retain
this
function
in
pigmented
organs.
Thus,
the
melanins
(
which
can
form
abiologically
)
may
be
the
oldest
evolved
system
for
defense
against
oxygen
radicals,
rather
than
SOD/catalase.
Free
radicals
are
produced
by
environmental
causes
such
as
light
or
ionizing
radiation.
However,
three
physiological
processes
can
result
in
extraordinarily
high
levels
of
radical
species.
These
include
the
mixed-function
oxidase
system
of
endoplasmic
reticulum,
the
NADPH
oxidase
system
of
inflammatory
cells,
and
the
presence
of
high
levels
of
autoxidation-mediating
charge-transfer
agents.
Production
of
activated
species
by
such
mechanisms
can
exceed
the
capacity
of
local
protective
mechanisms
and
produce
tissue
injury.
Inflammatory
cells
produce
active
species
of
oxygen
in
antimicrobial
defense
(1,2).
While
such
species
may
directly
damage
surrounding
tissues,
their
major
secondary
role
may
be
to
mediate
important
components
of
the
inflammatory
response.
For
example,
Figure
3
lists
some
of
the
inflammatory
immunomodulators
reported
to
be
affected
in
vitro
by
one
or
more
components
of
the
active
oxygen
system.
Inflammation
in
the
general
sense
comprises
the
whole
of
the
systemic
response
to
injury,
so
many
of
these
same
processes
may
also
occur
in
ischemic
injury,
for
example.
While
circumstantial,
the
list
includes
most
of
the
major
components
of
the
inflammatory
response
and
grows
daily.
Similarly,
antioxidants,
SOD,
and
catalase
have
significant
anti-inflammatory
properties
(3-5).
For
example,
Orgotein,
the
pharmaceutical
preparation
of
SOD,
is
used
in
veterinary
medicine.
It
is
reported
to
be
both
safe
and
effective
in
the
treatment
of
various
inflammatory
and
degenerative
lesions
in
man
(
3-4
).
The
action
of
many
other
antiinflamatory
drugs
may
also
involve
interactions
with
the
active
oxygen
system.4
Such
agents
may
act
by
interfering
with
the
action
of
phagocyte-produced
active
oxygen
species
on
one
or
more
of
the
systems
outlined
in
Figure
3.
The
role
of
active
species
in
the
inflammatory
response
may
also
explain
the
dermal
pigmentary
response
in
inflammation
(4).
Active
oxygen
species
may
also
have
a
role
in
endotoxin
shock,
burn-induced
plasma
volume
loss,
and
even
in
atherosclerosis
-
e.g.,
the
atherosclerotic
lesions
in
homocystinuria.
Likewise,
radical
mechanisms
may
play
a
role
in
stroke,
cerebral
edema,
and
spinal
cord
injury,
as
well
as
ischemic
injury
(
42
).
Drug
Toxicity
Radically
mediated
drug
toxicity
usually
occurs
by
one
or
both
of
two
main
mechanisms
These
are
(
1
)
production
of
activated
drug
metabolites
(
chiefly
by
the
microsomal
oxidation
system
)
and
(
2
)
production
of
active
species
of
oxygen,
a
process
often
involving
redex
cycling.
Examples
include
hepatotoxins
such
as
acetaminophen,
halothane,
and
carbon
tetrachloride
and
nephrotoxins
such
as
the
nitrofurantoins,
cis-platinum,
and
the
aminoglycoside
antibiotics.
Both
the
action
and
toxicity
of
important
antitumor
agents
such
as
adriamycin,
cis-platinum,
and
bleomycin
seem
to
depend
upon
production
of
active
oxygen
species
(4~45
)
and
often
involves
redox
cycling.
The
differential
toxicity
of
such
agents
to
tumor
cells
may
depend
upon
the
relative
paucity
of
antioxidant
defense
mechanisms
in
malignant
cells,
while
a
significant
part
of
their
organ
toxicity
may
be
a
consequence
of
the
paucity
of
antioxidant
defenses
in
the
extracellular
space
(4).
Fibrosis
A
variety
of
active
oxygen-generating
agents
can
produce
fibrotic
changes.
Examples
include
oxygen
itself,
paraquat,
nitrofurantoins,
and
bleomycin,
which
produce
pulmonary
fibrosis.
Radical-generating
agents
such
as
iron
and
copper
are
also
associated
with
liver
fibrosis
(
cirrhosis
)
and
fibrotic
changes
in
other
organs
such
as
the
heart.
The
induction
of
vitreous
scarring
by
interocular
iron
or
copper
is
also
well
known,
as
is
the
association
of
homocystinuria
with
fibrotic
lesions
of
the
arteries.
Figure
4A
shows
human
pulmonary
fibrosis
produced
by
exposure
to
high
levels
of
oxygen,
while
4B
shows
fibrosis
produced
by
nitrofurantoin.
Pulmonary
fibrosis
is
also
seen
in
such
diseases
as
asbestosis
and
cystic
fibrosis,
where
it
may
be
a
consequence
of
the
production
of
active
species
by
inflammatory
cells
and
perhaps
mucus.
As
an
illustration
of
the
commoness
of
radically-induced
pulmonary
fibrosis
to
nonclinicians:
both
pictures
are
from
randomly
assigned
autopsy
cases
done
by
the
author
on
a
general
autopsy
service
over
a
10-week
period
during
which
27
other
autopsies
were
done
-
two
others
of
these
were
bronchopulmonary
dysplasia
(
BPD
).
The
clinical
importance
of
radical
damage
to
lung
becomes
even
more
impressive
when
Adult
Respiratory
Distress
Syndrome
(
ARDS
)
and
its
permutations,
which
are
likely
mediated
by
production
of
active
oxygen
species
by
inflammatory
cells,
are
included.
Radical
production
by
ectopic
agents
may
induce
pathological
fibrosis
because
it
minics
the
nonpathogenic
activity
of
a
normal
modulator
system
linking
production
of
active
oxygen
species
by
inflammatory
cells
with
scar
formation
as
part
of
the
healing
process.
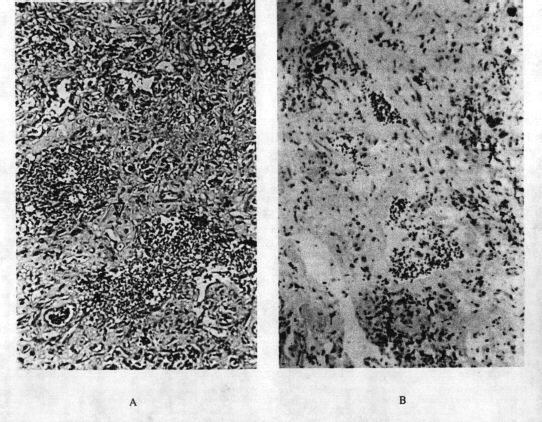
Figure
4:
Pulmonary
Fibrosis
FIGURE
4.
"Generic"
pulmonary
fibrosis.
A:
Interstitial
pulmonary
fibrosis
(
BPD
)
secondary
to
neonatal
oxygen
exposure
in
a
6-month-old
infant
girl.
BPD
is
a
common
sequela
in
premature
infants
given
oxygen
(
magnification
x
200)
and
B:
interstitial
fibrosis
associated
with
chronic
use
of
nitrofurantoin
for
urinary
tract
infection
in
a
62-year-old
woman
(
magnification
400X
).
In
both
cases,
normal
lung
is
almost
completely
displaced
by
interalveolar
(interstitial)
fibrosis
(
scarring
).
The
interstitial
space
is
normally
very
thin.
Other
oxygen
radical-generating
agents
such
as
paraquat
and
bleomycin
produce
a
similar
picture.
ARDS
(
or
"
shock
lung
"
)
is
another
pulmonary
disease
apparently
related
to
inflammatory
cell
production
of
active
oxygen
species
and
probably
oxygen.
Histologically,
ARDS
closely
resembles
the
early
stages
of
oxygen
or
paraquat
poisoning.
Both
autopsies
were
performed
by
the
author.
Charge-Transfer-Associated
Disorders
The
third
major
mechanism
for
endogenous
generation
of
activated
species
is
by
autoxidation
-catalyzing
charge-transfer
agents
such
as
copper,
iron,
or
manganese.
This
work
was
pioneered
by
Cotzias
and
co-workers
for
chronic
manganism.
Significantly,
the
concept
of
a
metal/neuromelanin/free
radical
interaction
was
part
of
the
basis
of
the
development
0f
levodopa
therapy
for
Parkinson's
disease.
The
quote
in
the
caption
for
Figure
2
is
appropriate.
To
summarize
this
area:
chronic,
elevated
systemic
levels
of
autoxidation-catalyzing.
melanin-binding
charge-transfer
agents
are
associated
with
combinations
of
characteristic
symptoms.
These
include
psychosis,
movement
disorders
(
dyskinesias
),
deafness,
pigmentary
abnormalities,
inflammatory/fibrotic
processes,
and
arthritis.
Significantly,
renal
tubular
lesions,
cardiomyopathies,
and
diabetes
can
be
associated
with
many
such
agents;
another
name
for
hemochromatosis
is
"
bronze
diabetes
",
while
many
diabetogenic
agents
are
notorious
radical
producers.
Likewise,
cardiomyopathy
with
consequent
heart
failure
is
a
common
cause
of
death
in
the
iron
storage
diseases.
Such
considerations
may
also
explain
the
correlation
between
nephrotoxicity
and
ototoxicity
with
drugs
such
as
the
aminoglycoside
antibiotics
and
cis-platinum.
Table
1lists
some
such
diseases,
the
associated
agents,
and
the
characteristic
symptomology.
The
correlation
of
radical-generating
agents
with
fibrotic
and
arthritic
symptomology
is
readily
explicable
in
terms
of
the
apparent
role
of
such
species
in
the
inflammatory
process,
as
outlined
in
Figure
3.
Similar
(
often
extracellular
?
)
mechanisms
may
hold
for
cardiomyopathy,
renal
tubular
impairment,
and
diabetes.
However,
the
correlation
of
such
agents
with
neuropsychiatric
symptoms,
while
long
known,
is
somewhat
harder
to
explain.
Some
interaction
with
melanin
in
the
inner
ear
and
midbrain
is
possible.
Such
agents
bind
to
melanin
by
charge-transfer
mechanisms
for
much
the
same
reason
that
they
catalyze
radical
oxidations.
Several
reviews
list
a
few
of
the
ways
in
which
active
processes
might
interact
with
neurological
diseases.
These
include
interactions
with
neurotransmitters,
their
effector
systems,
or
autoxidation.
Other
mechanistic
possibilities
include
relatively
nonspecific
damage
to
neural
tissues
and
interactions
with
neuro-
or
inner-ear
melanin
(4).
Again,
it
is
presently
impossible
to
select
from
among
such
possibilities.
As
in
the
case
of
inflammation,
it
is
likely
that
specific
mediator
processes
are
particularly
significant.
Perhaps
activated
processes
play
a
mediator
role
in
nervous
function
similar
to
that
which
they
apparently
play
in
the
inflammatory
process.
That
is,
active
species
may
be
neurotransmitters
in
the
same
sense
that
they
appear
to
be
cellular
and
immunomodulators.
In
this,
they
join
a
long
list
of
agents
(
e.g.,
monoamines,
cyclic
nucleotides,
and
the
prostaglandins
)
with
such
multiple
roles.
Later
note:
The
concept
that
free
radicals,
etc.
have
a
general
messenger
function
is
now
known
as
Free
Radical
(
or
Redox
)
Signalling.
TWO
EXAMPLES
OF
FREE
RADICAL-ASSOCIATED
DISEASES
Diseases
of
Purine
Metabolism
My
introduction
to
this
area
in
the
late
1960s
was
the
accidental
discovery
(
during
studies
on
the
Lesch-Nyhan
syndrome
)
that
uric
acid
and
other
purines
can
mediate
a
Fenton-type
reaction
with
peroxide,
as
well
as
act
as
antioxidants
and
cofactors
for
parotid
adrenalin
oxidase
(3).
Purines
also
catalyze
the
autoxidation
of
epinephrine
under
certain
conditions.
The
latter
may
involve
a
Fenton-type
reaction
with
peroxide
produced
by
adrenaline
autoxidation
(72).
The
realization
that
such
disparate
and
superficially
contradictory
properties
are
all
consequence
of
the
powerful
reducing
properties
of
purines
led
us
to
make
two
suggestions
(1)
That
the
choreoathetosis
found
in
the
Lesch-Nyhan
syndrome
is
but
one
more
case
of
the
association
between
dyskinesia
and
the
chronic
presence
of
charge-transfer
agents,
(4,9,10,24)
as
previously
noted
by
Cotzias
and
co-workers
for
chronic
manganism,
for
example,
and
(2)
That
the
physiological,
evolutionary,
and
pathogenic
roles
of
uric
acid
in
primates
are
explicable
in
terms
of
its
reducing
properties
--
for
example,
in
primate
evolution
uric
acid
has
been
substituted
for
ascorbate,
another
reducing
agent
with
both
pro-
and
antioxidant
properties
(32)
The
validity
of
such
hypotheses
is
supported
by
their
ability
to
predict
new
data
and
explain
old
observations.
For
example,
hyperuricemic
syndromes
present
variably
with
most
of
the
symptomology
associated
with
the
chronic
presence
of
charge-transfer
agents.
Likewise,
Lowrey
(6)
reports
a
hyperuricemic
syndrome
in
Dalmatian
dogs
which
is
associated
with
deafness
and
"
bronzing
"
and
even
responds
to
SOD
treatment
-
three
seemingly
unrelated
findings,
all
predictable
and
explicable
in
terms
of
activated
etiological
mechanisms.
An
obvious
corollary
is
that
hyperuricemic
syndromes
in
man
might
be
associated
with
pigmentary
abnormalities,
although
this
is
not
as
yet
reported.
Subsequently,
Rolfe
(55)
suggested
that
the
substitution
of
urate
for
ascorbate
might
explain
the
high
relative
resistance
of
man
to
ascorbate
depletion.
Many
workers
have
noted
the
antioxidant/reducing
properties
of
urate
in
relation
to
its
physiological
function
(4~34).
For
example,
10
years
after
our
initial
publication(32),
Ames
and
co-workers
rediscovered
the
possible
relationship
between
urate
and
ascorbate
in
primate
evolution
during
studies
on
the
antioxidant
properties
of
uric
acid
(33).
Like
the
melanins,
urate
may
also
act
as
an
antioxidant
by
binding
transition-series
metals
such
as
iron
and
is
apparently
a
better
antioxidant
and
much
poorer
pro-oxidant
than
ascorbate
(70).
There
is
even
good
evidence
that
urate
may
be
related
to
primate
longevity
through
its
antioxidant
properties
(34).
We
also
used
activated
mechanisms
to
explain
the
neurological
symptoms
of
the
Lesch-Nyhan
syndrome
and
the
evolutionary
role
of
urate
years
before
evidence
emerged
that
they
are
also
involved
in
inflammatory/arthritic
diseases.
It
now
seems
that
similar
processes
may
be
responsible
for
gouty
inflammatory
disease
(4,57).
Again,
there
are
many
possible
mechanisms
by
which
purines
could
mediate
inflammatory
processes.
For
example,
phagocytized
urate
likely
mediates
Fenton-type
reactions
with
granulocyte-produced
peroxide.
Binding
of
iron,
the
classic
mediator
of
Fenton's
reaction,
could
facilitate
(or
inhibit?)
such
processes.
This
could
explain
granulocyte
lysis
following
urate
crystal
ingestion
-
a
primary
pathogenic
process
in
gout.
Similarly,
purines
can
modulate
inflammatory
cell
function
by
various
other
activated
(
?
)
mechanisms
(4).
Thus,
both
the
physiological
and
evolutionary
roles
of
urate
are
readily
explained
by
its
antioxidant/reducing
properties.
On
the
other
hand,
the
pathogenesis
of
hyperuricemic
syndromes
may
involve
its
pro-oxidant
properties.
This
illustrates
the
often
paradoxical
problems
inherent
in
assigning
a
role
for
radical
mechanisms
in
human
disease.
For
example,
the
well-established
association
between
high
urate
levels
and
atherosclerosis
could
be
a
protective
reaction
(
antioxidant
)
or
a
primary
cause
(
pro-oxidant
).
Hemochromatosis
Iron
salts
are
the
classic
mediators
of
free
radical
processes.
As
Table
1
indicates,
hemochromatosis
and
other
iron
storage
diseases
are
but
two
examples
of
the
association
of
chronic
elevated
levels
of
charge-transfer
agents
with
characteristic
symptomology.
Significantly,
hemochromatosis
is
variably
associated
with
all
six
of
these
signs.
The
iron
storage
diseases
demonstrate
the
power
and
significance
of
recent
discoveries
in
free
radical
pathogenesis,
since
--
as
with
purinergic
syndromes
--
most
of
the
diverse
symptoms
of
this
class
of
diseases
are
potentially
explicable
in
terms
of
activated
mechanisms.
Later
note:
Increased
neuromelanin-bound
iron
is
found
in
Parkinson's
disease
For
a
good
review
of
the
pathophysiology
of
Parkinson's
Disease,
Go
Here
.
Conclusions
Electronically-activated
mechanisms
may
be
involved
in
many
of
the
most
basic
pathogenic
mechanisms,
some
of
which
are
listed
in
Figure
3.
In
fact,
active
species
seem
to
be
so
involved
in
the
ultimate
fundamental
common
pathway(s)
of
tissue
degeneration
that
the
expression
"
free
radical
pathogenesis
"
is
perhaps
redundant.
A
free
radical
etiology
of
disease
ultimately
involves
free
radical
involvement
in
symptomology,
for
which
there
is
abundant,
if
circumstantial,
evidence.
Nonetheless,
existing
protective
mechanisms
are
adequate
enough
that
active
species
can
be
used
for
certain
normal
physiological
processes.
Almost
certainly,
these
include
antimicrobial
defense
and
xenobiotic
metabolism.
Active
species
also
probably
act
as
mediator
substances
in
the
inflammatory
process
and
perhaps
even
as
neuromodulators.
It
follows
that
acute
radical
pathogenesis
normally
occurs
under
circumstances
of
extraordinary
radical
flux.
Such
conditions
include
inflammation,
radiation,
high
oxygen
tension,
and
xenobiotic
metabolism.
Similarly,
specific
common
symptomology
is
associated
with
extraordinary
levels
of
potentially
autoxidation-mediating,
melanin-binding
charge-transfer
agents
such
as
iron
or
urate
(
Table
1
).
In
particular,
as
Figure
3
outlines,
production
of
active
species
is
a
likely
primary
event
in
the
nonspecific
tissue
response
to
injury
(
inflammation
).
This
further
confounds
our
already
tenuous
ability
to
assign
a
role
for
active
species
in
specific
pathogenesis.
For
example,
is
the
protective
effect
of
SOD
and/or
catalase
against
radiation
or
antitumor
agent
toxicity
due
to
inhibition
of
the
primary
injury
or
to
inhibition
of
the
systemic
response
to
that
injury
?
Another
cogent
example
of
the
potential
pitfalls
of
circumstantial
evidence:
veterinarians
often
use
Palosein®,
the
veterinary
form
of
SOD,
to
ameliorate
injury
in
animals
struck
by
automobiles.
Obviously,
this
does
not
mean
that
motor
vehicles
produce
primary
injury
by
free
radical
mechanisms.
On
the
other
hand,
active
mechanisms
are
a
powerful
tool
for
explaining
normal
and
disease
processes.
It
has
already
been
noted
how
their
application
to
disorders
of
purine
metabolism
has
evolved
--
used
first
to
explain
the
neurological
features
of
a
very
rare
disease,
then
to
explain
the
unique
physiological
and
evolutionary
role
of
uric
acid
in
primates,
and
finally
to
explain
the
pathogenesis
of
purine-induced
inflammatory
disease
in
both
man
and
Dalmatian
dogs.
Also,
there
are
those
intriguing
hints
listed
in
the
text
and
the
associations
listed
in
Table
1,
some
doubtless
fortuitous.
If,
as
seems
reasonably
well
established,
active
species
act
as
immunomodulators,
why
not
also
neuromodulators
?
Thus,
psychosis,
dyskinesia,
pigmentary
abnormalities,
deafness,
and
inflammatory/fibrotic
syndromes
may
show
similar
etiologies.
This
has
important
therapeutic
consequences,
because
it
may
be
possible
to
control
some
radically
mediated
processes
pharmacologically.
Later
note:
For
examples
using
nitrone
and
nitroxide
spin
traps
and
spin
labels
to
treat
human
diseases,
see
stroke
.
I
hold
the
primary
patents.
One
nitrone
spin
trap,
"Cerovive",
AstraZeneca's
registered
trademark
for
disulfonyl-PBN
or
"
NXY-059
",
is
currently
in
Phase-3
clinical
trials
for
ischaemic
injury
in
stroke.
Finis
Keywords:
this
is
not
intended
to
make
any
sense
):
human
parkinsonism
parkinson's
disease
neuromalanin
melanin
iron
hemochromatosis
free
radical
spin
trap
spin
label
melanin
charge
transfer
lesch-nyhan
bromism
iodism
wilson's
disease
manganism
inflammation
neurofibrillary
tangles
fibrosis
nitrone
porphyria
n-oxide
prostaglandin
superoxide
dismutase
sod
hydrogen
peroxide
hydroxy
radical
reactive
ozygen
species
etiology
copper
transition
series
metal
redox
signaling
als
reperfusion
injury
cytokine
nkbeta
amorphous
semiconductor
organic
threshold
switching
electronic
properties
keywords
alopecia
balding
hairloss
redox
cellular
signaling
free
radical
superoxide
nitrones
nitrone
nitroxide
nitroxides
conductive
organic
polymers
semiconductors
dismutase
free
radical
minoxidil
peptides
peptide
propecia
antiandrogen
antiandrogens
drug
treatment
hair
alopecia
antiandorgens
polyacetylene
human
diabetes
alcaptonuria
antioxidant
homogentisic
phenothiazine
proxidant
vitamin
c
conductive
organic
metals
polymers
polymer
ascorbate
vitamin
aminoglycoside
tobramycin
gentamycin
chloroquin
cellular
signaling
radical
chloroquin
e
deafness
deaf
tardive
pigmentary
abnormalities
albino
ototoxic
diabetic
drug
aminoglycoside
waardenberg
cis-platinum
adriamycin
bleomycin
porphyria
musculoskeletal
ataxia
telangectasia
messenger
uric
acid
urate
iron
manganese
iodide
dalmatian
ataxia
redox
signalling
dyskinesia
psychosis
schizophrenia
dementia
vascular
stria
vascularis
substantia
nigra
midbrain
basil
ganglia
locus
ceruleus
pigmented
diffuse
alveolar
damage
stroke
reperfusion
homocysteine
injury
fenton
reaction
cytochrome
c
nitric
oxide
neuromelanin
phenylbutylnitrone
pbn
skin
myocardia
infarction
mitochondria
catalase
ards
glutathione
peroxidase
transmission
nkbeta
nxy-o59
copper
antiaging
iron
inos
cnos
neurotransmission.
No
inner
ear
lung
platelet
hair
interstitial
ards
dad
pbn
edrf
pulmonary
edema
asbestosis
asbestos
cardiac
atherosclerosis
heart
homocystinuria
active
oxygen
autoxidation
autooxidation.
Parkinson
disease
neuromalanin
melanin
hemochromatosis
iron
free
radical
spin
trap
spin
label
schizophrenia
melanin
iron
charge
transfer
Lesch
Nyhan
bromism
iodism
wilson
disease
waardenberg
dyskinesia
aminoglycoside
gentamycin
tobramycin
nephrotoxicity
stria
vascularis
inner
ear
manganism
inflammation
neurofibrillary
tangles
ataxia
musculaskeletal
telangectasia
fibrosis
deaf
deafness
melanoma
pigment
cell
reperfusion
injury
neurological
prostaglandin
superoxide
multinfarct
redox
cellular
signaling
dementia
atherosclerosis
myocardial
infarction
stroke
mi
mitochondria
dismutase
sod
superoxide
paraquat
hydrogen
peroxide
hydroxy
radical
reactive
ozygen
species
ros
etiology
copper
transition
series
metal
redox
cell
signaling
cytokine
nkbeta
amorphous
semiconductor
organic
threshold
switching
electronic
regrowth
alopecia
hair
loss
properties
antioxidant
proxidant
oxidant
oxidation
reduction.
Cancer
altzheimer's
beta
amyloid
nos
cnos
inos
disease
senile
dementia
pbn
tempol
tempo
drug
dmpo
nxy-059
nitrone
nitroxide
drug
treatment
polyacetylene
human.
diabetes
proxidant
vitamin
c
nitrosative
stress
ascorbate
vitamin
e
deafness
deaf
nephrotoxic
phenothiazine
pigmentary
abnormalities
albino
cell
signaling
ototoxic
redox
tubular
necrosis
gentamycin
tobramycin
signalling
aminoglycoside
cis-platinum
tobramycin
gentamycin
adriamycin
bleomycin
messenger
uric
acid
urate
hemochromotosis
proxidant
iron
manganese
iodide
dalmatian
hyperuricemia
ataxia
dyskinesia
psychosis
cytokine
homocysteine
heart
cardiovascular.
Sod
mimetic
dementia
musculoskeletal
vascular
stria
vascularis
parkinson.
Locus
caeruleus
substantia
nigra
midbrain
basil
heart
disease
arteriosclerosis
ganglia
locus
ceruleus
pigmented
nitric
oxide
nitrosation
stroke
nxy-059
nitrone
stroke
reperfusion
injury
nitric
oxide
myocardial
infarction
mitochondria
pathogenesis
free
radical
redox
cellular
signalling
schizophrenia
ataxia.
Home
As
summarized in Figure 3,
granulocytes and other
phagocytic cells
possess a membrane
NADPH oxidase,
which-takes reducing
equivalents from the
hexose monophosphate
shunt and transfers
these to molecular
oxygen to produce
superoxide and other
active oxygen species.
A further
myeloperoxidase
converts peroxide
produced in this system
to microbiocidal
products, probably
including hypochlorite
(2). Production of
activated products by
this system probably
plays a key role in
cell-mediated immunity
and microbiocidal
activity. There is
evidence for similar
systems in
T-lymphocytes,(15)
platelets,(6) and
mucus.(17) An NADPH
oxidase of
noninflammatory cells
may have a role in
mediating cyclic
nucleotide metabolism (
18-20 ).
Spin
Traps