Amorphous Semiconductor Switching in Melanins
Reprinted with permission from Science, vol 183, 853-855 (1974) Homepage. copyright 1974, American Association for the Advancement of Science.
Abstract: Melanins produced synthetically and isolated from biological systems act as an amorphous semiconductor threshold switch. Switching occurs reversibly at potential gradients two to three orders of magnitude lower than reported for inorganic thin films, and comparable to gradients existing in some biological systems. Of a number of other biological materials tested, only cytochrome c acted similarly, but at the high potential gradients reported for thin film amorphous semiconductors.
There has been a suggestion that the biological pigment melanin may qualify as an amorphous semiconductor (1). Recent experimental evidence has demonstrated that this material exhibits properties consistent with a more exotic form of an amorphous semiconductor, a threshold switch. Threshold switching has become a central issue in the development of amorphous electronic devices, but until now only inorganic materials have been demonstrated to possess these properties.
Synthetic melanins were produced both by the enzymatic action of mushroom tyrosinase (Worthington Biochemical) on tyrosine incubated in a buffer at pH 7.0 for 4 days. at 37C and by the autoxidation Of L dopa ( 3,4 hydroxyphenyl L alanine ) in IM NaOH for I week. The product was adjusted to a neutral pH, dialyzed against twice distilled water, and lyophilized:
Melanosomes were isolated from 8 g of human melanoma obtained at autopsy. The tumor material was strained through a 200 mesh grid to form a single cell suspension in a phosphate buffer, This suspension was then homogenized in a Dounce homogenizer, breaking the cell membrane but leaving the nuclear membranes intact. The homogenate was centrifuged at 10OOg for 10 minutes to remove the remaining intact cell nuclei and other cell debris. The supernatant was centrifuged at 50OOg for 10 minutes, and the new supernatant was again centrifuged for 30 minutes at 15,000g. The pellets from these centrifugations were then resuspended and layered on 30 to 50 percent sucrose density gradients in a Beckman SW 27 rotor and spun at 10,000 rev/min for 30 minutes; the gradients were fractionated and examined microscopically for their contents, The center one third of each gradient was found to contain particles in the size range 0.1 to 1.0 tan. The central fractions were ppoled and centrifuged at 50,000g for 4 hours to fdrm black pellets weighing approximately 20 mg, which were used for further study on melanosomes.
Melanin was also isolated by suspending 50 g of homogenized tumor in 6M HCI and centrifuging at 10,000g for 30 minutes. The pellet was subsequently washed with three rinses each of dimethylsulfoxide and acetone. In this case no effort was made to separate free melanin and melanosomes. The yield was approximately 50 mg. The melanin samples were cornpressed into cylinders 3 min in diameter and 0.1 to 10 min in length. The cylinder of melanin was compressed in a quartz tube (inner diameter, 3 min) between carbon, copper, or aluminum electrodes.
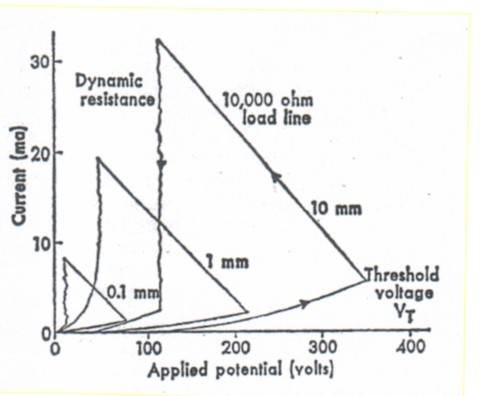
The electrical properties of all the melanin preparations were essentially the same. Figure I shows the typical electrical characteristics of melanin samples in series with a load resistor and an applied potential. As the applied potential is increased, the current through the melanin sample increases monotonically up to a voltage V, ( the threshold voltage), at which point the material switches.
Figure 1

Fig. 1. Current voltage properties of melanin prepared by autoxidation of L-Dopa for various sample thicknesses; copper electrodes were used. Data were obtained by using an X-Y plotter and circuitry as described in the text. The curves are typical of results obtained with other electrodes and melanins. Materials that do not switch (in. a sense, have no VT) either never leave the lower curve, or break down irreversibly. The current through a melanin sample, however, is dependent on its history, and will be given by the lower curve unless VT has been exceeded, in which case the dynamic resistance line applies. This process is reversible and is not a breakdown in the usual sense. The load line (negative slope) merely reflects the X- Y trace as the current and voltage adjust from the "off" to the "on" state and is inversely proportional to the value of the resistor in. series with the melanin sample; the potential therefore represents the voltage across the sample.
Most materials do not switch. In this case the current at a particular voltage is always the same, resulting in a single curve. Materials that switch exhibit two separate voltage current characteristics, the "on" and "off" states, At the point of switching (Vt), the X- Y recorder leaves the "off" curve and follows a. line whose slope is inversely proportional to the load resistor (the load line) until it reaches the curve charw2teristic of the "on" state, producing the triangular pattern in Fig. 1.
This sequence may be repeated in definitely; for example, a 10,000 ohm load resistor was sufficient to prevent a shift in the dynamic load line, even though V, was found to decrease about 20 percent on the first firing and then stabilize. [A decrease in V, by a factor of 5 to 15 has been reported in inorganic amorphous semiconductors on first firing; however, 50 percent was considered typical (2).] As Fig. I also shows, Vt, increases as a function of sample thickness, which indicates a dependence on the applied electrical field strength. Switching may also depend on the presence of absorbed water.
When samples were dried for 30 minutes at 200C, they would not switch until rehydrated and dried at room temperature. Rosenberg and co workers (3) have shown that the conductivity of the melanins (presumably in the "off" state) has a protonic component. Water, however, is also capable of lowering the activation energy for conduction, apparently as a result of local modification of the dielectric constant of the material. Since the current density in the "on" state is several orders of magnitude higher than that in the "off" state for the same applied potential, one can obtain an estimate of the protonic contribution as follows: approximately 10 mg of water must be dissociated to produce a current of 25 ma for 1 hour. A 30 mg sample (25 ing of melanin and 5 mg of H20) was switched 'son" and maintained at 25 ma for 3.5 hours, at which time the current was still being maintained at the initial voltage. This would require hydrolysis of over 30 mg of water, if 100 percent protonic conduction is assumed. The sample was found to exhibit switching properties at the end of this time. It appears that the protonic contribution to the conductivity in the "On" state is negligible, and the water probably acts by altering the local dielectric constant of the material.
We attempted to measure the switching time by adjusting the applied voltage to a value just below VT. At this potential, the material oscillated between the "on" and "off" states. The oscillations were viewed with an oscilloscope and found to be square waves with roughly a I msec lifetime and a rise time of less than 10 6 second.
The conductivity of dopa melanin and isolated melanosornes was relatively high (10 5 (ohrn cm) 11, resulting in a resistance of 104 ohms for a sample I min thick. The conductivity in the "on" state is increased by a factor of 100 to 1000. Hence in the "on" state melanin is a relatively good conductor. Using the same techniques, we investigated the electrical characteristics of a number of other biological molecules. Bovine serum albumin, mynglobin, lecithin, polytryptophan, bilirubin, and oxidized cholesterol were not observed to switch (at least at potential gradients below 5 X 105 volt/ cm). Equine cytochrome c switched at 4 X 105 volt/cm, three orders of magnitude higher than the potential gradients of melanins and comparable to those of inorganic amorphous semiconductors (4).
Switching phenomena in thin amorphous films and chalcogenide glasses have been extensively studied (4, 5). Since more information is becoming available on these materials, we wished to determine to what extent the results for melanins parallel those reported for some inorganic amorphous semiconductors. Switching behavior in these materials is usually reported for samples less than 10 lam thick and with potential gradients greater than 105 volt/cm (4). Melanins, however, switch at 3.5 X IG2 volt/cm and through at least I cm of material. ItT should be noted that this potential gradient exists in some biological systems.
Switching at low gradients. and through bulk samples poses interesting theoretical questions. However, the consistent appearance of melanin in living organisms at locations where energy conversion or charge transfer occurs (the skin, retina, midbrain, and inner ear) is of particular interest in view of the evidence for a role for melanin in such human disorders as parkinsonism, (6 8), schizophrenia (7), and deafness (8). The role of melanin in these disorders may be in some way related to its ability to function as an electronic device. This possibility is supported by the observation that the electronic properties of the melanin persist in intact melanosomes.
JOHN MCGINNESS, PETER CORRY, PETER PROCTOR
Physics Department, University of Texas Cancer Center, M. D. Anderson Hospital and Tumor Institute, Houston 77025
14 December 1973
Post-publication Note (2001) Another missed opportunity--- melanins give a flash of light when they switch-- clearly electroluminescence, though we did not completely understand its significance at the time.
References and Notes
I. J. E. McGinness, Science 177, 896 (1972)
2. M. P. Shaw, S. C. Moss, L. H. Stack, S. A Kostylev, Appl. Phys. Let. 22, 114 (1973).
3. B. Rosenberg and E. Postow, Ann. N.Y. Acad. Sei. 51, 162 (1969); M, R. Powell and B. Rosenberg, Bioenergetics 1, 493 (1970)
4). S. R. Ovshinsky it H. Fritzsche, IiEE (Inst. Elecir. Electron Eng.) Trans. Electron Devices ED 20, 91 (1973).
5. D. Adler"'in CRC Critical Review of Solid State Sciences (Chemical Rubber Company, Cleveland, Ohio, 1971), p. 317.
6. G. C. Cotzias, P. S. Papavasiliou, M. H. Van Woort, A. Sakamoto, Fed. Proc, 23, 713 (1964).
7. P. Proctor, Physiol. Chem. Phys. 4, 349 (1972).
8. N. G. Lindquist, Acta Radiol. Suppl. 325 (1973).
9. We thank M, Romsdal and S. Moss for their assistance in this work. Supported in put by PHS training grant CA 05099 and by AEC contract AT (40 H 2832.
Return to Organicsemiconductors.com
Viewers may view, Brouse, and/or download material for temporary copying purposes only, provided these uses are for non-commercial personal purposes. Except as provided by law, this material may not be further reproduced, distributed, transmitted, modified, adapted, performed, displayed, published, or sold in whole or in part without written permission from the publisher.
keywords: semiconductors organic metals device metal applications conductive polymers parkinsons melanoma deafness polyacetylene polymer semiconductor organic metals metal electroluminescent electrolumanesence polymers.