This 30+year-old paper may contain the first suggestion that radicals are cellular messengers. later dubbed "redox-signaling" ), If anybody has anything earlier, please contact me at drp@drproctor.com. so I can give them proper credit.
Other "lost" suggestions here are that homocysteine pathogenesis involves electron-transfer processes ( including oxidative stress ), that hyperuricemia involves oxidative stress, and that polyacetylenes such as melanin can be "doped" by charge-transfer agents (the proof of which won somebody else the 2000 Nobel Prize). Again, if this is wrong, please let me know.
See Harman for a primary antecedent. For a later version of this review, go here. For more on homocysteine and redox signaling, go here. For more on homocysteine and altzheimers dementia go here, here and here . For more on doped polyacetylenes go here.
Physiol Chem. & Physics 4 (1972) 349-360
ELECTRON-TRANSFER FACTORS IN PSYCHOSIS AND DYSKINESIA
PETER PROCTOR
Department of Physics, The University of Texas at Houston, M. D. Anderson Hospital and Tumor Institute, The University of Texas at Houston Graduate School of Biomedical Sciences, Houston Texas
(Received May 17,1972)
SUMMARY
In man, chronic elevated systemic levels of compounds possessing electron-transfer properties are typically associated with one or more of a triad of characteristic. signs. These are psychosis, dyskinesia, and abnormalities in pigmentation. The possible in vivo interactions of such compounds are discussed.
INTRODUCTION
While there is strong indirect evidence for biological factors in the etiology of the heterogeneous group of psychiatric disorders known as "schizophrenia (1-3) and also in the etiology of various movement disorders such as parkinsonism (4) no single etiological factor has as yet been well defined in either class- of disease.
A step in this direction is the work of Cotzias et al.(5) reiterated by Curzon(4), -who noted an intriging correlation between the chronic presence of substances having electron-transfer properties, e.g., in phenothiazine treatment or manganese poisoning, and the appearance of movement disorders (dyskinesias) in man. It has been noted (6) that disorders associated, with the chronic presence of electron-transfer agents are also typically characterized by either or both of two additional symptoms- schizophrenaia-like psychosis or pigment . abnormalities.: The occurrence, significance, and possible etiology of such coincident signs in relation to the common electronic property of the compounds involved in their production are discussed.
ELECTRON-DONOR ASSOCIATED SYNDROMES
An electron -donor may be described as a substance which shares an electron ( or rather a statistical part of one ) in a -charge-transfer complex with an electron acceptor. Complete electron~transfer results. in a reduction~oxidation reaction, the electron donor being-oxidized and the electron acceptor being reduced.(7). A single-electron transfer often results in unpaired electrons on both the electron donor and the electron acceptor, both of which become free radicals.
A summary of pertinent data, concerning a number of syndromes associated with the presence of electron-transfer agents is given in Table 1. Column I lists the disorders, along with references to reviews of them. Column 2 lists the electron-transfer agent(s) involved, and the third column lists an electron-donor-ability-related, semiempirical quantity known as the energy of the highest occupied molecule orbital (HOMO) energy.
| Syndrome | Compound | HOMO energy | Reduce PTA ? * | Psychosis | Dyskinesia | Pigment | Catalyzes Oxidation of .... |
| ascorbate, (a) | 0.49 | yes (11) | -- | -- | -- | linoleic acid (41) | |
| tocopherol | 0.58 | ? | -- | -- | -- | -- | |
| Lesch-Nyhan Syndrome (b) | Uric acid | 0.18 | Yes (11) | yes (59) | choreoathetosis (59) | ? | epinephrine (see figs 1&2) |
| Dopa treatment | Dopa | 0.62 | Yes | yes (11) | choreaoathetosis | ? | TMPD(29), autoxidizes (c) |
| Phenothiazines | chlorpromazine (e.g.) | -0.11 | ? | see (67) | parkinsonism, tardive dydkinesia (d) | Yes | |
| Alcaptonuria (71) | Homogentisic acid | 0.63 | yes (71) | ? | parksonsonism | Yes (71) | autoxidizes |
| Homocystinuria (73) | homocysteine thiolactone | 0.07 | yes (11) | yes (73,74 | ?, see comment e | hypo ? (73) | autoxidizes |
| Hyperthyroidism | e.g, thyroxine (f) | 0.49 | ? | yes (75,76) | choreoathetosis | yes (75) | glutathione |
| Iodism | iodide | ref 29 | -- | yes (79) | iodate (80) causes retinal hyperpigmentation | TMPD (29), (c) | |
| Bromism (81) | Bromide | ref 29 | -- | yes (81) | tremor & ataxia (81) | yes (81) | ? (29) |
| Wilson's disease (g) | copper | ref (30) | -- | yes (52) | yes (52) choreoathetosis | yes | many compounds (30) |
| Manganese poisoning (h) | manganese ores | ref (5) | -- | yes (85) | yes (84) | ? | e.g., epinephrine |
| Hemochromatosis (87) | iron (i) | ref (30) | -- | occasionally (87) | ? | yes(87) | many compounds (30) |
| Acute intermittent porphyria (88) | porphobilinogen | 0.43 | ? | yes (88) | choreoathetosis | ? | autoxidizes (88) |
* PTA = Phosphotungstic acid.
Comments:
a. Reducing agents block O2-dependent inhibition of
phenyalanine hydroxylase by epinephrine (97)
b. Suggestion of some pigmentation in brain of
Lesch-Nyhan patient (66)
c. TMPD is tetramethylparaphenyenediamine
d. Tardive dyskinesia resembles choreoathetosis
e. " Hint of Parkinsonism " in father of (
homocystinuric) patient (74)
f. Activity of thyroid hormones may be related to
electorn-donor properties ( (29, 78 )
g. Wilson's (90) obesrvation that the symptoms of
chronic lenticular degeneration are much like those of neonatal
jaundice is significant in light of electron-transfer properties
of the bile pigments (91).
h. Unweathered (i.e., unoxidized ) manganese ores most
toxic (86). Chronic mercury poiosoning is also associated with
psychosis (92), dyskinesia (82, 92), and hyperpigmentation (93).
i. Hallorvorden-Spatz disease associated with
abnormal iron deposition. (4).
1) Phenothiazines inhibit the oxidation of reduced NAND by
melanin (94).
The HOMO energy values listed were calculated on an IBM 7094 computer using a program prepared by Novak and Furlong for the calculation of molecular orbital indices using the Huckle approximation. HOMO energy values range around 1.0 for most aromatic compounds. The electron-donor ability of a compound increases as the HOMO energy becomes more negative. While comparisons of HOMO energies of compounds not in the same homologous series are dangerous, a compound with a HOMO energy of 0.50 or less is generally considered to be a " good " electron donor. The HOMO energies of ascorbate and a-tocopherol, compounds whose physiological roles are generally considered to be related to their electron-donor, i.e., reducing, properties are included for comparison. Since HOMO energies can be calculated only for compounds having delocalized electrons (6) none are given for iodide, bromide, etc. The electron-transfer properties of these compounds are considered in the references given. Using these criteria, the compounds listed can be categorized as good to excellent electron donors. The negative value for chlorpromazine signifies an " antibonding orbital " and implies particlarly good- eletron-donor properties for this compound.
. Column 4 provides an. empirical check on the electron-donor properties implied in column 3. The classical colorimetric method for the clinical determination of uric acid is based upon the unique ability of that compound to directly reduce phosphotungstic acid (11). The similar electron-donor properties of L-dopa and its metabolites account for the artifactual rise in serum uric acid levels found with L-dopa therapy (12).
The next three columns of Table I summarize the main point of this paper; namely, that in man chronic elevated levels of substances having single-electron-transfer properties are typically associated with one or, more significantly, more than one of a triad of characteristic symptoms which include psychosis, dyskinesia, and pigmentary abnormalities. As the table shows, all three symptoms are reported in Wilson's disease and in thyrotoxicosis. In the Lesch-Nyhan syndrome and in L-dopa treatment, psychosis and dyskinesia are predominant. Likewise, chronic administration of the phenothiazines is accompanied by both dyskinesia and hyperpigmentation. Similarly, psychosis and hyperpigmentation are found in patients with bromism, while psychosis occurs independently in patients with homocystinuria.
Because the compounds associated with the triad of psychosis, dyskinesia, and pigmentation abnormality have nothing in common but a rather unusual electronic property ( i.e., the ability to participate readily in electron-transfer processes ), the hypothesis seems tenable that there may be electron-transfer factors in the etiology of these disorders. For this reason, it is worthwhile to consider the possible common in vivo interactions of electron-transfer agents in order to clarify the nature of such factors. Particularly pertinent interactions are those involving melanin, the catalysis of nonenzymatic autoxidations, cofactor properties, general reducing properties, the activation of psychoactive (13-15) plasma protein components, and interactions with biological semiconductors.
MELANIN
As Table I shows, for example, abnormalities in pigmentation tend to be associated with the chronic presence of substances having charge-transfer properties. The question arises as to whether such pigmentary changes reflect, indirectly or not, analogous processes occurring in the brain. Are these pigmentary changes the indirect visible manifestation of some ongoing primary process such as altered monoamine metabolism or free radical or excited-state production, or do they reflect changes in brain melanin metabolism which directly produce the associated signs of psychosis and dyskinesia?
Skin melanin presumably functions as a screen for UV fight. (16). However, the human brain has highly pigmented, nonilluminated areas, such as the substantia nigra and the locus coeruleus , (17) in which melanin must have some other function, e.g., a sink for free radicals,(18) a redox buffer,(9) or a biological device for dissipating the energy of the excited states of biological molecules (20).
Melanin is also a good electron acceptor, (14), has semiconductor properties, (14) and might be expected to form charge-transfer complexes with compounds having electron-transfer properties. Cotzias et al (5) noted that both naturally occurring and drug-induced dyskinesias occurred only in species which possessed visible melanin in the substantia nigra . These authors explained the paradoxical ability of such free radical-forming agents as the phenothiazines to induce and to relieve dyskinetic symptoms by suggesting that there is an optimum level of melanin free radicals in the brain, and that increasing or decreasing this level can result in dyskinetic symptoms. Such phenomena also might be related to the process of changing melanin alternately from a conductor to an insulator by progressively filling conduction bands by electron transfer (21).
Dr P sez: Speaking of "doping" of melanin. In 1974, we reported that melanin can act as a semiconductor " bistable switch ". That is, melanin is an " active device ", i.e., one whose electrical conductivity can be modulated by an electric field, such as in a transitor. Your computor is just an array of bistable switches.
Three years later others reported that another polyacetylene can be chemically-modified to a similar high conductivity state by iodine. Note that " iodism " is listed in this review. They received the 2000 Noble Prize in Chemistry for this discovery. There is no evidence that the prize committee was aware of our earlier, more advanced work, though it was published in a major journal, Science. For more on this, go here.
As noted, we also found that melanin can be doped chemically. Though clearly the Nobel committee differs, we considered this trivial. It was eight years before anyone else reported anything similar ( a field-effect transistor ) using a conductive organic polymer. Our "gadget" is now in the electrical collection of the Smithsonian as the putative first organic electronic device.
Similarly, electron donors are known to play several different roles in the synthesis of melanin. For example, catechols such as L-dopa are presumably the substrates from which melanin is synthesized and, as we shall see, electron donors may act as cofactors for melanin synthesizing Systems (22,23). For example, the similarity between the symptoms of the Lesch-Nyhan syndrome and., the. side effects of L -dopa therapy (24) might be explained by examining either the affinity of the purines and the catechols for brain melanin. or the possible action of the purines as cofactors - in the peroxidase-catalyzed (22,23) synthesis of L-dopa and, ultimately, melanin. ( For other ways in which alterations in the metabolism of melanin or its precursors might affect CNS function see references 25-28.)
CATALYSIS OF AUTOXIDATIONS
Another property of electron-transfer agents is their general ability to catalyze non-enzymatic autoxidations by participating in a charge-transfer complex with molecular oxygen, which becomes " activated " by accepting an odd electron (29--31). Cooperative oxidations also occur in which an organic electron donor serves to maintain a catalytic transition-series metal in the active reduced state (30). Since the substrate-independent initial step in catalysis-that is, the formation of such a complex-is the critical one, the catalysis of the oxidation of one compound should imply the ability to catalyze the oxidation of others. Column 8 of Table I notes oxidations catalyzed by the various compounds listed.
(An example of such a reaction-- the uric acid-catalyzed autoxidation of epinephrine-- is given in Figures I and 2.) The common catalytic ability of thyroxine, bromide, and iodide may be relevant to their common pharmacologic properties (32,33).
Further, such oxidations involve single-electron transfers and would thus give rise to free radicals. In this case, the work of Polis et al (34) on the psychoactivity of the free radical derivatives of several biological molecules is relevant, as is the possible role of melanin as a sink for free radicals(8), and the role of free radicals in initiating melanin synthesis (35).
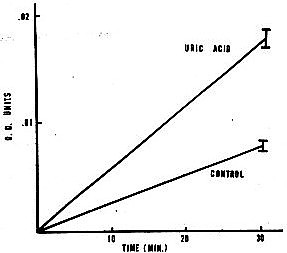
Figure 1. Spectrophotometric assay of catalysis of epinephrine oxidation by uric acid.

Procedure: Increase m optical density at 485 nrn followed using a Gilford model 240 spectrophotometer with a Heath model EUW 20-A servorecorder, 0.00 to 0.10 O.D. full-scale. Control: 1.0 x 10-4 ML-epinephrine in 0.012 M phosphate buffer (pH 7.4). Uric acid: control + 3.5 x 10-4 M uric acid (5.9 mg% urate). Standard errors are given.
Figure 2. Radiometric assay of catalysis of epinephrine autoxidation by uric acid.
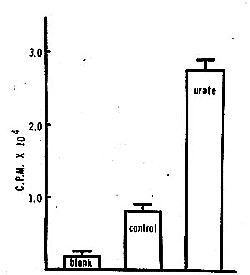
Procedure: (Modification of the method of Axelrod.38) Control: 1.0 ml of 0.1 M phosphate buffer (pH 7.4) + .18 Mg MgS04 + 0.1 mg phenylisopropylhydrazine + 0.3 gCi DL-epinephrine-4-3H (0.96 mCi/gM). Urate: control + 3.4 x 10-4 uric acid (equivalent to 5.7 mg % urate, a physiological concentration). Samples are incubated for 10 min at 370C, at which time is added LO ml of 1.0 M borate (pH 10. 0) and 5.0 ml cold toluene: isoamyl alcohol (3:2, v/v). Mixture is shaken for five min and then centrifuged for 10 min at 900 x g. Three ml of the organic phase is then counted in a suitable liquid scintillation medium (" Diatol "). Axelrod (38) has found that most of the radioactivity in the organic phase after such a procedure is in the form of the 0-phenylisopropylhydrazine derivative of adrenochrome. Standard errors are given. The blank represents the amount of radioactivity extracted without incubation.
Dr P note : Uric acid levels are correlated with risk of dying from heart attack . " Free radicals " have been postulated as a possible mechanism.
Also, Heikkila and Cohen (36) have shown that the damage to noradrenergic nerve terminals produced by 6-hydroxydopamine-- an autoxidation product of dopamine which Stein and Weise (37) relate to the etiology of schizophrenia-- is in fact caused by peroxide, a product of the autoxidation of the former compound.
COFACTOR PROPERTIES
Another common property of strong electron donors is their ability to act as cofactors for certain oxidative enzymes, either by maintaining a transition-series metal in the reduced state at an active site or by providing activated oxygen as an' electron acceptor (29). For example, L-dopa acts as a cofactor in the peroxidase-catalyzed oxidation of tyrosine to L-dopa; in addition, through its autoxidation, it serves as a possible, source of peroxide (22).
(Peroxidase, in addition to possibly initiating some brain melanin synthesis, may also play a role in brain catecholamine production.(22,23) ). Likewise, chlorpromazine, monophenols (38) and uric acid (39) all stimulate parotid adrenaline oxidase, while epinephrine and serotonin may have similar cofactor roles in the oxidative synthesis of the prostaglandins (40).
REDUCING PROPERTIES
Strong electron donors can also inhibit oxidation by either reducing oxidized species, terminating free radical chain reactions, or by competing with an oxidizable substrate, e.g., sulfhydryl groups, for activated -oxygen. Thus, Haase (41) found that ascorbate could catalyze the autoxidation of linoleic acid at low concentrations while inhibiting it at higher ones. Presumably, whether electron-transfer agents catalyze or inhibit oxidations is dependent upon the amount of oxygen dissolved in solution.
ACTIVATION OF PSYCHOACTIVE PLASMA PROTEIN FACTORS
Bergen (42) reported that Plasma Globulin- Precipitate ( PGP ), a psychoactive plasma protein from schizophrenics, is greatly activated in vitro in the presence of moderate levels of reducing substances such as mercaptoethanol or ascorbate. The fact that PGP is a lipoprotein and that higher concentrations of electron donor tend to suppress its activity suggests that the electron donor may be catalyzing an oxidation, for example, to a lipid free radical.
SEMICONDUCTOR INTERACTIONS
The interaction of electron-transfer agents with biologically important semiconductors has been noted in relation to melanin. However, as Szent-Gyorgyi (13) and Cope (43) point out, many other biologic materials, such as proteins, bimolecular lipid membranes, and DNA, have quite appreciable semiconductor properties which may be relevant to their functions. Electron-transfer agents might alter the electronic properties of such materials by inserting electrons into a conduction band, as in the case of N-type semiconductor, or into holes, as in the case of a P-type semiconductor. For example, Pant and Rosenberg showed that the magnitude of the photocurrent through the Fe+++ -complexed-oxidized cholesterol bimolecular membrane--- a P-type semiconductor-is greatly diminished in the presence of reducing agents. Similar results were -found with a mixture of chlorpromazine (45)and melanin.
As -has been considered, a combination of these effects ( i.e., filling of a conduction.band to produce an insulator followed by the insertion of carrier electrons into the next highest empty band to restore conductivity ) might be relevant to the ability of certain electron donors, e.g., phenothiazines' or bromides (4) to induce the very symptoms they are-effective against (20,21).
MISCELLANEOUS INTERACTIONS
Other interactions are also possible. Brillouin (51) has proposed that band splitting resulting from the interaction of degenerate (equal energy) orbitals on two electron donors, such as a protein and an aromatic amine, might elevate the highest occupied orbital to such a degree an electron transfer to a suitable electron acceptor could occur. The importance of charge-transfer phenomena in enzyme action has been considered in refs. 14, 47-49. Furthermore, transition-series metals such as selenium may also facilitate electron transfers to electron acceptors other than oxygen. Also, molecules are better participants in charge-transfer reactions in the excited state than in the ground state (7) In reference to the de Sanctis-Cacchione syndrome, Lamola (51) has reviewed evidence for the existence of excited-state species in the brain. What has been said about electron-transfer agents is also applicable to the reactions of such excited-state species.
CONCLUSIONS
As the discussion shows, the possible common interactions of electron-transfer agents with biological systems are varied and can be completely opposite. It is likely that more than one type of interaction might take place in vivo. For example, the dyskinesia associated with Wilson's disease ( which occurs at a stage at which there is no visible brain damage ) may be caused by the affinity of copper for brain melanin. Later damage to the basal ganglia and to the liver may be the result of copper-catalyzed peroxidation of membrane lipids. Numerous other more or less indirect processes are possible, e.g., disruption of lysosomal function by altering ascorbate metabolism (53), degradation of DNA ( by cysteine(54) or by diphenols (55) ), or melanin-related alterations in pineal function. Cooperative interactions, such as a transition-series metal operating in conjunction with a purine to produce a psychoactive protein free radical, are also possible.
Dr P sez: Recurrent reduction of a catalytic transition-series metal, etc. by a reducing substance is now known as " redox cycling ". A possible example is the potentiation of beta-amyloid neuronal toxicity by copper and homocysteine. Similarly, the ability of free radicals and so forth to act as specific cellular messengers ( including neurotransmission ) is now dubed " redox signalling ".
Further, it is uncertain whether electron-transfer processes have a role in the etiology of "idiopathic" movement disorders or in the schizophrenia psychosis itself. Cotzias et al.(5) and Curzon (4) have reviewed the evidence for such processes in the dyskinesias. Similar factors may be relevant to the increased incidence of both movement disorders (56) and hypermelanization (25) found in schizophrenic populations prior to the use of phenothiazines.
Likewise, certain electron donors, such as the sulfur-containing amino acids, (57, 58), dopa,(59) or disulfiram (60) can cause or exacerbate schizophrenic symptomatology. Similarly, serum uric acid levels have. been related to degree of illness in some schizophrenics (61) while there is a high incidence of psychosis reported in the relatives of a homocystinuric patient(62). The role of electron donors as in vitro activators of a psychoactive plasma protein peculiar to schizophrenics (42) has been mentioned. Such data are consistent with the role of electron-transfer factors in the etiology of at least some cases of the rather heterogeneous group of diseases labeled as " schizophrenia ".
In summation, two statements on the possible role of electron-transfer processes in psychosis and in the movement disorders can be made with some degree of assurance. First, as Cotzias and his co-workers suggest, brain melanin appears to have a role, indirect or not, in the etiology of certain movement disorders. Whether it also has a role in the etiology of the psychosis associated with several of those movement disorders or with other psychoses remains to be shown. Second, the electron-transfer reactions of the compounds associated with the group of disorders noted in this paper are predominantly single-electron transfers and thus quite likely involve the production, at least transiently, of free radical or excited-state species. Perhaps it is the chronic presence of such electronically activated species which, in some as yet undefined manner, forms the etiological basis of the psychosis-dyskinesia-pigmentation abnormality complex.
ACKNOWLEDGMENT
I wish to thank Drs. N. B. Furlong, R. T. Harris, B. T. Ho, G. F. Farrell, B. Novak and J. E. McGinness for their assistance. This work was partially supported by USPHS grants CA 05099, 00254, and by grant R- 171 from the Robert A. Welch Foundation.
REFERENCES
1. L. H. Heston, Brit. J. Psychiat., 112, 819 (1966).
2. J. D. Rainer, in Schizophrenia: Current Concepts and Research, D. V. Siva Sanker, Ed., PJD Publications, Ificksville, N. J., 1969, p. 303.
3. J. R. Smythies, F. Benington and R. D. Morin, in Schizophrenia: Current Concepts and Research, D. V. Siva Sanker, Ed., PJD Publications, Hicksville, N. J., 1969, p. 496.
4. G. Curzon, Int. Rev. Neurobiol., 10, 323 (1967).
5. G. C. Cotzias, P. S. Papavasiliou, M. H. Van Woert and A. Skamato, Fed. Proc., 23, 713 (1964).
6. P_ Proctor, Lancet 1, 1069 (197 1).
7. B. Pullman, in Molecular Biophysics, 0. Waala , Ed., Academic Press, New York, 1965, p. .117.
8. W. Novak and N. B. Furlong, Texas Rep. Biol. Med., 2 7, 1041 (1970).
9. J. P. Malrieu, in Molecular Basis of Some Aspects ofMental Activity, 0. Waalas, Ed.-, Academic Press, -New York, 1967, p. 83.
I.G. G. Karreman, 1. Isenberg and A. Szent-Gyorgyi, Science, 130, 1191 -(1959). 11. W. T. Caraway, CZin. Chem., 15, 7-20 (1-969).
12. M. T. Cawein and J. Hewins, New Eng. J. Med., 281, 1489 (1969).
13. A. Szent~Gyorgyi, Bioelectronics, Academic Press, New York, 1968.
14. B. -Pullman and A. Pullman, Quantum Biochemistry, Academic Press, New York, 196 3.
15. L. Brillouin, in Horizons of Biochemistr , M. Kasha and B. Pullman, Eds., Academic Press, New York, 1962, p. 295.
16. M. Seiji and M. S. Itakura, J. Invest. Dermatol., 47, 507 (1966).
17. M. Bazelon, G. M. Fenichel and J. Randall, Neurologjv, 17, 5 12 (196 7).
18. B. Commoner and J. L. Fernberg,Proc. Nat. Acad. Sci. U.S.A., 47,1374 (1%1).
19, V. Horak and J. R. Gillette, MoL PharmacoL, 7, 429 (197 1).
20. J. E. McGinness and P. H. Proctor, in preparation.
21. J. E. McGinness, Physics Today, 23, 81 (1971).
22. M. R. Okun, B. Donnellan, W. F. Lever, L. M. Edelstein and N. Or, Histochemie, 25, 289 (1971).
23. G. S. Bayse and M. Morrison, Biochim. Biophys. Acta, 244, 77 (197 1).
24. P. Proctor-and J. E. McGinness, Lancet 11, 1367 (1970).
25- A. C. Greinoi-, 6an. Psychiat. Ass. J., 15, 433 (1970).
26. Z. L. Hegedus and M. D. Altschule, Arch. Biochem. Biophys., 126, 388 (1-968).
27. M. D. Altschule, in Molecular Basis of Some Aspects ofMental Activity, Vol. 2, 0. Waalas, Ed., Academic Press, New York, 1967, p. 415.
28. G. C. Cotzias, M. H. Van Woert and L. M. Schiffer, New Eng. J. Med., 276, 374 (1967).
29. G. Cilento and K. Zinner, in Molecular Associations in Biology, B. Pullman, Ed., Academic Press, New York, 1968, p. 309.
30. L. L. Ingraham, Comprehensive Biochemistr , Vol. 14, M. Florkin and E. H. Stoltz, Eds., y Elsevier, New York, 1966, p. 424.
31. 0. Hayaishi, in Proceedings of the Robert A. Welch Foundation, Vol. 15, 197 1, in press.
32. E. Gruenstein and J. Wynn, J. Theor. Biol., 26, 342 (1970).
33. S. Varrone, E. Consiglio, and 1. Covelli, Eur. J. Biochem., 13, 305 (1970).
34. B. D. Polis, J. Wyeth, L. Goldstein and J. Graedon, Proc. Nat. A cad. Sci. U.S.A., 64, 755 (1%9).
35. M. A. Pathak and K. Stratton, in 7he Biological Effects of Ultraviolet Radiation, F. Urbach, Ed., Pergamon, New York, 1969, p. 207.
36. A. Heikkila and G. Cohen, Science, 172, 125 7 (197 1).
37. L. Stein and C. D. Wise, Science, 171, 1032 (197 1).
38. J. Axelrod, Biochim. Biophys. Acta, 85, 247 (1964).
39. P. Proctor, Thesis, University of Texas Graduate School of Biomedical Sciences, Houston, 197 1.
40. C. J. Sih and C. Takeguchi, reported at 3 1 st Int. Congr. Pharmaceut. Sci., Washington, D. C., 1971.
41. G. Haase, Ph.D. Dissertation, University of California at Davis, University Microfilms No. 69-16,351 (1968).
42. J. R. Bergen, Res. Commun. Chem. Pathol. Pharmacol., 1, 403 (1970).
43. F. W. Cope, Advan. Biol. Med. Phys., 13, 1 (197 1).
44. H. C. Pant and B. Rosenberg, Photochem. Photobiol., 14, 1 (197 1).
45. A. M. Potts and P. C. Au, Aggressol*e, 9, 225 (1968).
46. M. W. P. Carney,Lancet 11, 5 23 (197 1).
47. S. Shiffin, in Molecular Associations in Biology, B. Pullman, Ed., Academic Press, New York, 1968, p. 323.
48. M. Shinitzky and E. Kiaxhalski, in Molecular A ss6ciation in Biology, B. Pullman, Ed., Academic Press, New York, 1968, p. 361.
49., M - A. Slifkin and J - G. Heathcote, Molecular A ssociations in Biology, B Pullman, Ed., Academic Piess, New York, 1%8, p. 343.
50. G. N. Scbrauzer and W. J. Rhead, Experientia, 27, 1070 (197 1).
51. A - A. Lamola, Biochem. Biophys. Res. Commun., 43, 893 (197 1).
52. A. G. Bearnl- in The Metabofic Basis ofInherited Disease, J. B..Stanbury, J. B. Wygaarden, D, S. Fredrickson, Eds., McGraw-Hill-, New Yotk--- 1966
53. J - A. Edgar, Nature, 22 7, 24 (1-970Y.-
S4. H. S. Rosenkranz and S. Rosenkranz, Arch. Biochem. Biophys., 146, 483 (197 1).
55. K. Yamajugi, H. Murakami and M. Shinozuka, Z. Frebsforsch., 73, 195 (1970).
56. D. Goldman, Dis. Nerv. Sys., 29 suppl. 83), (1968).
57. W. Pollin, R_V. Carden and S. S. Kety, Science, 133, 104 (1%1).
58. J. Spaide, H. Tanimurai, R. Gunther and H. E. Himwich, Fed. Proc., 25, 626 (1966).
59. G. G. Celesia and G. Baff, Arch. Neurol., 23, 193 (1970).
60. J. M. Scher, J. Am. Med. Assoc., 20.1, 1051 (1967).
61. C. C. Pfeiffer, V. Iliev, L. Goldstein, E. H. Jenny and R. Schultz, Res. Commun. Chem. Pathal. Pharmacol., 1, 247 (1970).
62. N. A.Carson, D. C. Cusworth, C. E. Dent, C. M. B. Field, D. W. Weill and R. G. Westall, Arch. Dis. Childh6od, 28, 425 (1963).
63. W. L. Nyhan, Fed. Noc., 27, 1034 (1968).
64. C. F. Farrell, personal -communication.
65. W. L. Nyhan, J. A.- James, A. J. Teberg, L. Sweetman and L. G. Nelson, J. Pediat., 74, 20 (1969).
66. D. Hoefhavi, Fed. Proc. 27, 1042 (1968).
67. M. F. Halaz, J. Form and A. S. Marrozzi, Science, 164, 569 (1-969).
68. G..E. Crane, Aggressologie, 9, 209 (1968).
69. S. Carruthers, Brit. Med. J. 1 3, 5 7 2 (197 1).
70. A. C. Greinor and G. A. Nicholson, Lancet, 11, 1165 (1965).
71. B. N. LaDu, in The Metabolic Bd~is of Inherited Disease, J. B. Stanbury, J. B. Wyngaarden and D. S. Fredrickson, Eds., McGraw-Hill, New York, 1966, p. 303.
72. M. Sandler, F. Karourn and C. R. J. Ruthven, Lancet, 11, 770 (1970).
73. T. Gerritsen and H. k. - Waisman, in The Metabolic Basis of Inherited Disease, J. B. Stanbury, J Wyngaarden and D. S. Fredrickson, Eds., McGraw-Hill, New York, 1966, p.' 420.
74. A. C. Kaeser, R. Rodnight and B. A. Ellis, J. Neurol.-Neurosurg. Psychiat., 32, 88 (1969). 175. W. Locke, Med. Clin. N. Amer., 51, 915 (1967).
76. J. Durell, L. S. Libow, S. G. Kellan and R. 1. Shader, Res. Publ. Ass. Res. Nerv. Ment. Dis., Vol. 43, Williams and Wilkins, Baltimore, 1967, p. 387.
77. W. Heffron and R. P. Eaton, Ann. Intern. Med., 73, 425 (197 1).
78. J. Mauchamp and M. Shinitsky, Biochemistry,8, 1554 (1968)
79. S.S. Ketz, in The origins of Schizophrenia, J. Romano, Ed., Exerpta Medica Foundation, New York, 1967, P. 35.
80. A. M. Potts, Dis. Nerv. $ys., 29 (suppl.), 15 (1968).
81 M. W. P. Carney, Lancet, 11, 5 23 (1971).
82 A. H. Greenhouse, Arch. Intern. Med., 117, 389 (1%6).
83 M. L Lew, G. T. Strickland, C. C. Wang and T. S. Chen, J. Amer. Med. Ass., 211, 238 (1970).
84. H. A. Rosenstock, D. G. Simons and J. S. Meyer, J. Amer. Med. Ass., 217, 1354 (197 1).
85. K. L5ffelholz and H. Scholz, Experientia, 26, 637 (1970).
86. J. Rodier, Brit. J. In& Med., 12, 21 (1955).
87. M. Pollycove, in The Metabolic Basis of Inherited Disease, J. B. Stanbury, J. B. Wyngaarden and D. S. Fredrickson, Eds., _McGraw4iill, New York, 1966, p. 7 80.
.88. R. Schmidt, in The Metabolic Basis of Inherited Disease, J. B. Stanbury, J. B. Wyngaarden and D. S. Fredrickson, Eds., McGraw-Hill, New York, 1966, p. 813.
89. C. J.Witkop, Adv_ Human'. Genet., 2, 61 (1971).
90. S.A. K. Wilson, Brain, 34,.395 (1912).
91. B. Pullman'-and. A. Pullman, Quantum Biochemistry, Academic-Press, New York" 196 3, p. 427.
92. J. W. A. Turner, Lancet, 1, 66 1 (195 5)
93. . K. Burge and R. K. Winkleman, Arch. Dermatol., 102, 51 (1970).
94. M. H. Van Woert, Noe. Soc. Exp. Bial. Med., 129, 1%5 ~ 196 8).
95. S. H. Snyder and C. R. Merrill, Proc. Nat. Acad. Sci. U.S.A., 54, 258 (1965). U.S.A., 67,-62 (1970).
96. S. Kang and J. Proc. Nat. Acad. Sci. 67, 62 (1970)
97. C. Bublitz, Biochem. Pharmacol., 20, 2543 (1971).
"Cerovive" is the trademark of AstraZeneca for the stroke drug pbn disulfate.
Keywords: parkinsonism parkinson's disease neuromalanin homocysteine melanin hemochromatosis free radical spin trap spin label melanin sharge transfer Lesch-Nyhan bromism iodism wilson's disease manganism inflammation neurofibrillary tangles fibrosis prostaglandin superoxid dismutase sod superoxide hydrogen peroxide hydroxy radical reactive ozygen species etiology copper transition series metal redox signaling cytokine nkbeta amorphous semiconductor organic ros threshold switching electronic nitric oxide peroxynitrite properties antioxidant proxidant oxidant oxidation reduction cancer altzheimer's disease senile dementia pbn tempol tempo dmpo nxy-059 nitrone nitroxide.
drug treatment polyacetylene human diabetes proxidant vitamin c ascorbate vitamin E deafness deaf phenothiazine pigmentary abnormalities albino ototoxic drug aminoglycoside waardenberg cis-platinum adriamycin bleomycin messenger uric acid urate iron manganese iodide dalmatian ataxia dyskinesia psychosis organic metals schizophrenia dementia vascular stria vascularis substantia nigra midbrain basil ganglia locus ceruleus pigmented stroke reperfusion injury fenton's reaction cytochrome c nitric oxide reduction oxidiation myocardial infarction mi mitochondria organic metal catalase glutathione peroxidase transmission neurotransmission.inner ear lung platelet interstitial ards dad pulmonary edema asbestosis asbestos cardiac atherosclerosis heart homocystinuria active oxygen autoxidation autooxidation.
Schizophrenia parkinsonism parkinson's disease neuromalanin melanin hemochromatosis free radical spin trap spin label melanin sharge transfer Lesch-Nyhan bromism iodism wilson's disease conductive polymer polymers copper manganism inflammation neurofibrillary tangles fibrosis prostaglandin superoxide dismutase sod superoxide hydrogen peroxide hydroxy radical reactive ozygen species etiology copper transition series metal redox signaling cytokine nkbeta amorphous semiconductor organic threshold switching electronic properties organic metals metal antioxidant proxidant oxidant oxidation reduction homocystinuria atherosclerosis homocysteine thiolactone..
cancer altzheimer's disease senile dementia pbn tempol tempo dmpo nxy-059 nitrone nitroxide drug treatment polyacetylene human diabetes proxidant vitamin c ascorbate vitamin E deafness deaf phenothiazine pigmentary abnormalities albino ototoxic homocysteine aminoglycoside cis-platinum adriamycin bleomycin messenger uric acid urate iron manganese iodide dalmatian ataxia dyskinesia psychosis schizophrenia dementia vascular stria vascularis substantia nigra midbrain basil ganglia locus ceruleus pigmented stroke reperfusion injury nitric oxide myocardia infarction mitochondria pathogenesis free radical.